A reader writes:
Hello there Mr. Dan. I stumbled across your site whilst googling "can you get hurt making a potato battery". Yep, I googled that.
I (clearly) know little about the electronics/cathode/anode world... but could answer lots of questions about other things non electrical. :)
In planning my son's birthday party, I am considering a potato battery station (sounds odd for a party, but trust me, it fits with the theme).
I have seen several Youtube videos with instructions and examples, some done by children. My main question before I go buy a bag o potatoes and seek out the copper wiring aisle of Walmart is: Can children be hurt doing this? Yes, us grown-up types will be there too, but is there anything I should be concerned about?
Partying Mom
It is theoretically possible to kill yourself with potato batteries, but the chance of a kid managing to achieve this is much, much lower than the chance that one of them will fall over and crack his/her skull in your bathroom, and you probably won't lie awake at night worrying about that.
I could just leave it at that, but of course I won't. This is because I think an understanding of the basics of electrochemistry, which is what potato batteries are all about, is something that all modern humans should have, even if they never put it to use.
You should know why it's warmer in the summer (it's surprising how many people incorrectly say "because then we're closer to the sun", which, even if it were true, would make summer happen at the same time for both the northern and southern hemispheres...), you should know how tax brackets work, and you should also know the basics of the technology that envelops modern humans so completely that we hardly notice it at all.
Sorry, didn't mean to lecture you. This is just something I'm rather passionate about.
Getting back to potato batteries: The power output of an individual potato, or lemon, or what-have-you, "battery" is extremely low, which is why there are few-to-no things you can power from one spud with two pieces of dissimilar metal in it.
"Battery" is in quotes up there because one tuber and two bits of metal are a single electrochemical "cell"; technically, it's not a "battery" unless it has more than one cell in it. (So, of the things sold in the supermarket as "batteries", AAs and Cs and Ds are cells, but 9V or 6V batteries, composed of six and four 1.5-volt internal cells respectively, really are batteries.)
The open-circuit voltage of any electrochemical cell is determined by the electrode potential of the materials you use for the electrodes. If you build the usual kind of potato battery with copper and zinc electrodes (like, a copper or copper-plated coin, and a zinc-plated galvanised nail), each cell will have an open-circuit voltage of 1.1V, but a current capacity into a short circuit of less than a milliamp.
The larger the surface area of the electrodes, the higher the current capacity will be. But even with really big electrodes you'll probably only get half a milliamp into a short circuit - and the more of the cell's current capacity you use, the lower its output voltage will be.
(For comparison, I just grabbed a rather old but unused off-brand "super heavy duty" - meaning, carbon-zinc, not even alkaline - AA cell out of my Drawer Of Many Batteries, and it still reads more than 1.6 volts open circuit, with a short-circuit current capacity of more than 1.5 amps. Here's a PDF datasheet for an Energizer carbon-zinc AA; they've got a sub-site devoted to these things.)
If you make multiple potato batteries and put them in series and/or parallel, you can increase the voltage and/or current capacity of the whole battery, respectively. Two cells in series (both of which can be stabbed into the same potato; just connect the copper of one cell to the zinc of the next) and you get 2.2 volts open circuit and the same miserably tiny current capacity. Two cells in parallel, and you get 1.1 volts but double the current capacity. Six cells, wired up as series strings of three with the two strings in parallel with each other, and you get 3.3 volts and double current capacity. And so on.
(Many people seem to find the concept of series and parallel circuits tricky to grasp. It's another of those bedrock pieces of information about the world that I urge everyone to learn, though, because it explains a great deal of everyday electrical things. Why does one bulb dying in a string of old Christmas lights kill the whole string? Because they're ten or twenty 12V bulbs {depending on your local mains voltage} wired in series to connect directly to the mains. Why, in contrast, can you have a couple of things turned on and a couple of things turned off all plugged into the same powerboard and have everything work? Because the powerboard's outputs are in parallel!)
Getting back to your actual question, this is how you could, if you tried very hard, kill yourself with a potato battery. 30 milliamps across the heart has a pretty good chance of stopping it, and even lower currents have upon occasion been fatal. Kids might be more susceptible, too; I don't know.
Even sweaty skin is a good enough insulator that sundry low-voltage current sources aren't dangerous - grab the terminals of a 12V car battery with bare wet hands and you probably won't even feel a tingle, though a tiny current really will be flowing through your arms and across your chest. But if you stab probes into yourself, into your hands or preferably into your chest right on either side of the heart, then an array of potato batteries big enough to deliver tens of milliamps really could, if connected to the electrodes, kill you.
(One reason why high voltage can be especially dangerous is that it can spark a hole right through the skin, giving it access to your wet salty conductive innards.)
Given, of course, that this particular means of death starts out with stabbing yourself, you could simplify the process by just stabbing your heart directly.
Hence: Not worth worrying about.
(There's also an outside chance that you could poison yourself by eating a potato or lemon or whatever that's been used as a battery for a while, because it'll now be contaminated with various metallic salts. It probably wouldn't do more than make even a small child slightly ill, though, presuming he or she somehow managed to choke the vile-tasting thing down. This situation is even less likely to happen than chest-stabbing, unless you use some particularly delicious fruit instead of a potato or lemon.)
The great problem with potato-battery demonstrations in the past was not, of course, kids somehow killing themselves, but that it was very difficult to do anything with the extremely feeble output of such a battery. Turning even a tiny motor, or lighting even a grain-of-wheat incandescent bulb, was impossible without a ridiculous number of cells. Getting a feeble glow from a grain-of-wheat bulb rated for 12 volts and 80 milliamps could perhaps be done with as few as 50 potato cells, though I suspect you'd need a hundred or more.
So potato batteries usually ended up doing something lame like powering a pocket transistor radio with a piezoelectric earpiece, which is a feat that you can more impressively achieve with no battery at all.
Today, you could similarly fail to impress the youngsters by potato-powering one of those little LCD clocks and kitchen timers that're meant to run from a couple of button cells. Two or three potato cells in series might, at a stretch, be able to run one of those. A far better target, though, is lighting a light-emitting diode (LED).
A modern high-intensity red or amber LED will only want about two volts and a couple of milliamps to light dimly, and will be quite impressively bright at only 10mA. Ten parallel strings each containing two potato cells ought to be enough to give a pretty bright light, and each two-cell "string" could be only one potato.
Here's a red LED...

(image source Flickr user trvance)
...just barely glowing from only three copper/zinc lemon cells in series...

(image source Flickr user s8)
...and here's an excellent example of multiple cells in one lemon...
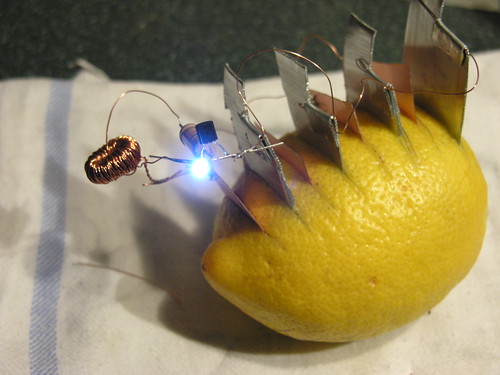
(image source Flickr user s8)
...which works extremely well because it's cheating, and using a simple four-component circuit (counting the LED) called a "Joule Thief", which I learned about years ago on the excellent Web site of the inimitable Big Clive.
I recommend you provide sufficient spuds and/or lemons, electrodes and alligator-clip leads to make lots of cells, and also provide a grab-bag of water-clear high-intensity LEDs so the kids don't know what colour they've got until they get it to light up.
A lot of LEDs will not cost you a lot of money. I find it mind-blowing that the going price on eBay for a pack of a hundred mixed waterclear high-intensity LEDs has, for some time now, been under five US bucks, delivered. I suggest you get 5mm LEDs, not the 3mm ones that're the absolute cheapest, because the smaller ones are a bit fiddly even for kids' hands.
(I don't actually need any more LEDs, but I just felt morally obliged to buy this hundred-5mm-LED pack, from this seller, for $US2.99 delivered. At this price you could use these things, which were a miracle of the age in the 1970s and have for years now been revolutionising a significant portion of the lighting industry, as notice-board pins. They are literally cheaper than thumbtacks. Even the ones with three different-coloured dies and an invisibly minuscule controller chip built in cost damn close to nothing.)
You should play with this stuff yourself before the party, so you can introduce the kids to the series/parallel idea, and help them if they don't know to chain the cells nose-to-tail (copper to zinc or zinc to copper, not copper to copper or zinc to zinc), and also see which way round you have to connect the LEDs to make them work. (They're light-emitting diodes; they only work one way around. Long leg positive.)
It would also be a really good idea to get the finest, cheapest digital multimeter eBay has to offer, so you don't have to rely on licking the ends of wires to estimate how many volts your potatoes have managed to make. Every home should have a crappy ten-buck yellow plastic multimeter; you may not use it often, but it can be very handy at times. (Put it in the kitchen drawer with the screwdriver, the hammer, the random screws and washers and the polycaprolactone.)
Depending on age and disposition, the kids may figure this all out for themselves, of course. LEDs only work one way round, a battery setup that'll light a 1.8V red LED probably won't light a 3.6V blue or white one, a setup that'll light a blue LED may very satisfyingly turn a red one into...

...a friode, you can series- and parallel-wire LEDs as well as batteries...
While you're shopping for quantum-physics miracles on eBay for three cents each, you could add a couple more things that used to be super-tech and are now super-cheap: Lithium coin cells, and rare-earth magnets.
2016 (20mm diameter, 1.6mm thickness) and 2032 (3.2mm thick) coin cells aren't as cheap as LEDs; if you buy them in a supermarket or pharmacy you can pay dollars for one. Again, though, just hit eBay and you can find fifty for less than 15 US cents each.
Rare-earth magnets can be even cheaper. If you restrict this search to Buy It Now items more suited to the impatient, you can get twenty 8mm-diameter 1mm-thickness neodymium-iron-boron disks for less than ten cents each; hundred-packs drop it to about seven cents apiece.
Why am I suggesting you buy these items?
Because you can light an LED by just pressing its legs to either side of a coin cell...

(image source Flickr user spike55151)
...and if you put LEDs (preferably diffused 10mm ones, but any with legs will work), coin cells and magnets together, you get...

(image source Flickr user c3o)
..."LED throwies".

(image source Flickr user chopsueyphoto)
Which are easy to make, and awesome.
Psycho Science is a regular feature here. Ask me your science questions, and I'll answer them. Probably.
And then commenters will, I hope, correct at least the most obvious flaws in my answer.