Alarm clocks are awful. Get one of these instead.
Even if your alarm clock is one of those Zen alarm clocks with melodious metal chimes, or it's your phone playing New Age music at gradually increasing volume, an alarm clock is still not offering you anything. It's just invading your rest and causing you to start your day with a little slap of sadness and irritation, arguably made even worse by the snooze button's empty gift of a few more minutes of half-sleep. Which you'll probably only spend trying to integrate your interrupted dreams with wakefulness.
(It is, at least, pleasant when I realise for the umpteenth time that I am not, in fact, still in high school.)
Or you may just lie there, living in fear of the return of the cursed alarm.
I shudder to think how much human misery those millions of little morning insults have added up to, over the centuries since humans first invented a water clock that would make a noise at a particular time.
Presuming you can't just rearrange your life so it doesn't matter when you get up, the best option in the pursuit of timed wakefulness is, clearly, a butler. A butler who brings you a cup of tea, even as he murmurs his apology for the regrettable necessity that you be conscious.
(The celebrated Stephen Fry alarm clock seems to no longer be in production, and it would also appear that more people paid for them than actually, strictly speaking, received a product in return. One can only hope that the Bible-verse version of the same product is similarly defunct. I would very much like to see a combination of the two.)
Failing that, what you want is a teasmade.
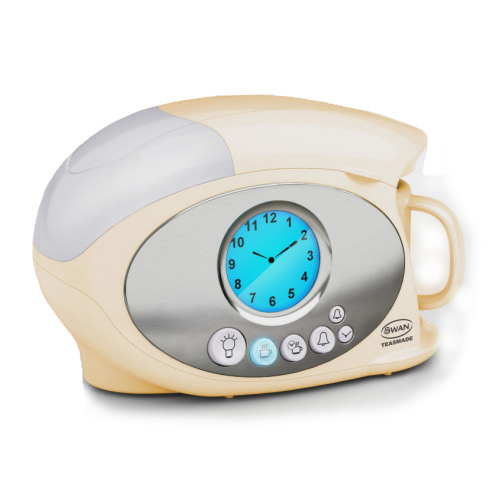
This one's mine. It's not a particularly elegant or collectible example, but it does the job.
The modern teasmade - the term has become a genericised trademark, in Britain at least - is essentially an electric kettle controlled by an alarm clock. When the alarm time is reached, the kettle element turns on, and a few minutes later boiling water is delivered to the tea-leaves.
Or to anything else you put in the little teapot, for that matter. If you want alarm-clock instant soup or Bovril or anything else you can prepare by putting it in a pot and pouring boiling water on it, you can have that too. (Might be a bit of a challenge eating soup out of the little teapot, though, if there are bits in it too big to exit the spout. You could also make a hot-milk-based beverage in a teasmade. But possibly only once.)
Most Automatic Food Machines, especially the ones that look like the coolest thing in the universe, have problems. They don't work in the first place, or they work only for a little while without unfeasible amounts of maintenance, or they're uncleanable, or their sole desire is to maim or murder their operator.
For every good gadget like the blade meat tenderiser or Aeropress coffee maker, there are a dozen crappy As Seen On TV wastes of time and money.
(There's also a small sub-category of wonder food gadgets that can only work by breaking laws of physics.)
A teasmade is not like that.
It is easy for a tired person to set up of an evening, it does what it's supposed to do without fuss, it has no moving parts except the control buttons, and its cleaning requirements are close to zero.
(Classically, you are never meant to do anything more than rinse a teapot; the accumulating tannin stains are supposed to make the tea taste better, though I'm pretty sure that claim doesn't stand up to double-blind testing. If you've got unusually hard water or use a teasmade for a long time then you may also need to clean lime-scale out of the boiling vessel and tube, but just running the teasmade with water and vinegar in the boiler should take care of this.)
The earliest alarm-clock tea-makers were created in the late nineteenth century. They were shiny and clockwork, with alcohol burners and a match-striker or other similarly implausible mechanism to light the burner when the alarm went off. These devices were less of a threat to life and limb than one might imagine, but were still less than entirely convenient to operate, and also rather expensive. And, to be fair, if there's one thing that'll wake you up even more effectively than a nice cup of tea, it's a fire on your bedside table.
The modern teasmade is electric, safe and reliable, and quite cheap. I bought mine used on eBay in 2003, and it cost me only $AU37.85 delivered. That was an unusually good deal - one just like it is on ebay.com.au as I write this, for $AU90 plus delivery - but working and pretty-safe-looking used teasmades routinely sell for well under $US100 delivered, and you can get a brand new one for less than 60 UK pounds delivered within the UK, $AU160-odd delivered to Australia, or around $US165 delivered to the States. Or less, if you buy the version with no radio, of which more shortly.
And yes, American buyers are likely to have voltage problems, because the teasmade is largely unknown outside the UK and as far as I know nobody makes a 110-volt one. More about that in the "buying one" section, below.
How it works
As you can see in the above picture, the modern teasmade really is essentially just a combination of an alarm clock - often, as in mine, a clock-radio - and an electric jug. It's quite easy to use.
The alarm clock in my teasmade works in the same way as every cheap plastic clock-radio. You set the time, you set the alarm, you tune the radio, and you select how you want the thing to wake you up.
In addition to the standard clock-radio options of an awful alarm noise or a tinny radio, though, my teasmade lets you select "tea" alone. You will then be awakened by the sound of boiling water, and the smell of a mildly caffeinated beverage.
My teasmade is rated at 600 watts at 240 volts, which is on the low side by electric-jug standards; here in Australia our mains electricity is a nominal 230 volts and a usual actual 240, so electric jugs with a power rating of 2000 watts or more are common.
My teasmade's water capacity is only about 650 millilitres - that's about 2.6 metric cups. Or a couple of good-sized mugs, or more than three dainty little teacups. The 600-watt heater takes about seven minutes to boil this full capacity; proportionally less if you don't fill it completely. You should of course take account of this boiling delay when setting the alarm time.
The initial heating process is quiet; it'd probably wake me up if I were sleeping without earplugs, since I'm a pretty light sleeper, but most people would sleep through it. The part where the boiling water is transferred to the teapot, though, is quite dramatically loud, and should be an effective alarm for most people all by itself.
The reason for the noise is the way in which my teasmade, like pretty much all others, transfers the boiling water from kettle to teapot. When you put the filling cap back on the boiling vessel, the boiler is sealed except for a metal tube that goes almost to the bottom of the vessel, and arches over to point at the middle of the lid of the...
...distinctive hole-topped little teapot. (If you find a junk-shop teapot that looks like this, you now know where it came from.)
When the water boils, the pressure of the steam pushes the water through the tube and into the pot. It takes no more than ten seconds for my teasmade to transfer a full pot-worth of water through the rather narrow tube. Hence the noise. When the reservoir's empty, it heats above the boiling point of water and a thermostat shuts off the heater. (There's also a switch that disables the heating element if the teapot isn't in place, so night-time absent-mindedness will not result in an unconfined spray of boiling water the next morning.)
If you need more of an alarm to wake you up then, ideally, you'd be able to set the horrible alarm noise or irritating radio station of your choice to go off when the water transfers, or even after the tea's had a few minutes to steep. But my teasmade can't do that; the alarm/radio goes off when the heating element turns on, at which point a single cup of tea is at least five minutes away, and a full pot is at least seven.
Some teasmades have more sophisticated alarm settings, so the alarm can go off when the boiling is completed, not when it starts:
OK, that alarm takes us straight back into the Land of Horrible Awakenings. But at least there is tea.
Going along with its cheap-clock-radio nature, my teasmade has no backup battery, and reverts to that good old flashing "12:00" and no memory of previous settings if there's even a momentary power cut. You can solve this problem by running the teasmade, and for convenience also your bedside lamp, from a small uninterruptible power supply. A pretty beefy UPS will even be able to run the tea-making element; a cheap one won't be able to do that, but will at least ensure continuity of timekeeping if the element doesn't try to click on from UPS power.
I'm hardly an authority on teasmades, though; there are a lot of different models, even if you disregard the pre-electric W. Heath Robinson versions.

(Image source: Flickr user James Mooney)
Here's a Goblin with a removable boiler, as well as teapot, presumably for ease of filling.

(Image source: Flickr user James Mooney)

(Image source: Flickr user Martin Deutsch)
I wish mine had a TEA NOW button.

(Image source: Flickr user leo.j.turner)
Another picture of the same model of teasmade, or at least one with the same buttons. It appears to have won an award. Good for it.

(Image source: Flickr user MarkyBon)
This one just screams Fawlty Towers.

(Image source: Flickr user gruntzooki, a.k.a. Cory Doctorow)
Another just like it, and some others, on display in the London Science Museum. It's apparently circa 1945.
The integrated lamps can be rather nice:

(Image source: Flickr user ebbandflo_pomomama)

(Image source: Flickr user Simon Harriyott)
This one looks as if it ought to be mystifying Jacques Tati in Play Time.
A simple search for "teasmade" (which may or may not correctly geo-target to your country; here the same search is on eBay UK, and here it is on ebay.com) gets 20 relevant hits on eBay.com.au as I write this, plus a few isolated teapots and a Bjork remix with "Teasmade" in its title.
There are some decent deals there, but I probably got my teasmade so very cheaply - under $AU40 delivered - because it was described as "Alarm clock/radio with teapot -RARE", which barely describes it and is almost impossible to search eBay for. I've no idea how I ever found it.
Even if all you throw into the eBay search box is some generic "tea maker" sorts of terms, it's pretty much impossible to filter out umpteen ordinary electric jugs, teapots with infusers in them, teapot-shaped kitchen timers and so on. Here's my best effort at making such a search across the whole of eBay.com.au with possible geo-targeting to other eBay sites; if that doesn't work, here's one for ebay.co.uk and here's one for ebay.com.
The easiest way to get a teasmade today is to just buy one new. For a while I think this may have been impossible unless you found a dealer with "new old stock", but now it's quite easy to buy a Swan teasmade online.
You may or may not care for the Swan's magic-lantern styling and LCD analogue clock, but on the plus side, you know the appliance hasn't been sitting in someone's garage for fifty years, maturing into a truly world-class fire hazard.
The Swan teasmades list on their site for £79.99 (about $US128 or $AU124, as I write this) ex delivery. They don't ship outside the UK, though.
The Swan teasmade is also on sale at this Union-Jack-waistcoat of a site, which is very excited to announce that the "Teasmade Classic is now £48.99 and the Radio Teasmade is now £69.99!". But they, also, only ship within the UK.
There are Swan teasmades on eBay; a US buyer could get the basic no-radio model for $US132.05 plus a mildly suspicious only $US6.00 for shipping, and an Australian shopper could get the same model for $US142.05 delivered.
That's not cheap, but at least the Aussie shopper would only need a plug adapter to connect a UK-sourced teasmade to Australian mains power.
(Until quite recently, it was normal for UK appliances to come with a power cable that terminated in bare wires, because the UK contained an incompatible mixture of the old BS 546 and new - in the sense of "after World War II" - BS 1363 wiring and plug standards. You had to buy a plug separately and screw it onto the cable yourself, or get someone in the shop to do it for you if you were a wuss. Nowadays BS 1363 is dominant enough that I think pretty much all UK appliances come with a BS 1363 plug moulded onto the end of the cable. Chopping that plug off and replacing it with one to suit your own country's mains, so you don't have to use an adapter, is unwise if you don't know what you're doing, but is legal in most countries.)
If you live in the USA, Canada or some other 110-to-120-volt country, though, you have a problem. Some teasmades wired for 110V are alleged to exist, and converting one wouldn't be an insoluble problem for an electronics hobbyist or repair-person, but you ain't gonna get one off the shelf.
So there's nothing stopping someone in the Americas from buying a teasmade from the UK or Australia, but it'll be the wrong voltage and you'll have to run it from a quite beefy step-up transformer.
Your beefy step-up transformer will very probably be an autotransformer, and very probably come with a piece of paper listing a wide variety of devices they strongly recommend you never plug into it on account of autotransformers' poor isolation qualities. A teasmade is likely to fall into at least two of those forbidden categories.
That said, two-pin sockets and cheater plugs do not yet seem to have killed most of the American population, and those are more straightforwardly dangerous than a teasmade running from a step-up transformer. Modern teasmades also have no exposed metalwork, so you're not really living too dangerously if you use one from a step-up transformer. I'd do it. But I am known for making poor decisions.
You can also get fully electrically isolated step-up transformers; they're more expensive, but solve the safety problems. And if your American house has a 240-volt circuit for a clothes-dryer or other high-powered electrical appliance, you can just plug the teasmade into that. (Running an extension cord from the one 240V outlet in the bathroom all the way to your bedroom may negate the safety benefit.)
The frequency of US 240V will be 60Hz instead of the 50Hz the teasmade expects, which will cause old-style electric clocks to run six-fifths as fast as they should. If you get an old teasmade with an analogue clock then there's a good chance it depends on the mains frequency to keep time, and will thus be essentially useless from the wrong frequency. You could run it from a frequency converter, but by now we're getting well out into the crazy-weed.
As long as there's no mains-synchronous clock in your teasmade, a different mains frequency shouldn't be a problem. A newer teasmade with a digital clock will very probably have a quartz oscillator that's immune to mains frequency changes.
Alternatives
By this point, American shoppers intrigued by the teasmade idea but disinclined to subscribe to British Appliance-Fancier Monthly will probably be thinking there must be a simpler way to do this. I mean, you could just plug an electric kettle into a timer switch and get something approximating the same functionality.
It occurs to me that if you get a coffee-maker that has a timer function, put tea leaves in it in place of coffee (and, if you want to get fancy, also replace the paper filter with a mesh strainer screen), you could get very close to a teasmade's functionality without all of the international-voltage bother.
The design of the typical modern no-moving-parts bubble-pump coffee-maker (which, incidentally, uses as a pump the same sort of device that propels a pop-pop boat)...
...is not ideally suited to making tea, but it'd more or less get the job done. A coffee-maker may not quite make Tea According To Orwell, but I'd drink it.
(Oh, and cheap drip coffee makers' primary purpose appears to be to make coffee snobs almost as apoplectic as percolators do, but a teasmade actually makes pretty close to optimal tea. It doesn't pre-heat the teapot, which is a mark against it, but it does deliver really scalding water onto the tea leaves, which is generally agreed to be Correct. Coffee benefits from being made with less-than-boiling water; tea does not. The fact that water boils at only 174°F at 20,000 feet {79°C at 6100 metres} is clearly a far greater problem for British mountain-climbers than any piffling shortage of oxygen.)
Cheap coffee-makers with timers require you to reset the timer every night, because they can't tell whether there's already coffee in the carafe or not, and want to avoid disasters that a forgotten full carafe could cause the next morning. (Leaving the water reservoir empty shouldn't be a problem, though, because even the very cheapest of coffee-makers should have a reliable overheat cut-off.)
You'd think you could get a coffee-making teasmade-analogue with an actual alarm-clock brewing function for a reasonable price, but I'm not sure if you can. I think you can do it with expensive plumbed-in models like this one, but few mere counter-top models seem to have such a feature.
I think this inexpensive Black & Decker model may qualify, though. I managed to find a manual for it online and it does seem to have a repeating alarm-coffee function. If you definitely know of such a thing, please do tell us all about it in the comments.
(On the subject of different approaches to the problem, check this out. Once again, some concept designer has supported my previously-expressed opinion of the breed, in this case by reinventing the teasmade and making it much, much worse. Apart from apparently being carefully designed to set itself on fire from the middle out, this concept design expects you to, first thing in the morning, drink tea out of a hemispherical E-Z Spill(TM) cup with no handle. This design also requires you to put a tea bag in cold water the night before and let it sit there for hours before the water is heated, which I can only presume will cause the ghost of Queen Victoria to manifest, reach into your chest and crush your worthless heart.)